Have you ever wondered how nutrients move from your blood to your cells, or how your body gets rid of waste products? The answers lie in the fascinating processes of diffusion and osmosis, two fundamental concepts in biology. I remember vividly the first time I encountered these concepts in high school biology. Our teacher set up a simple experiment using dialysis tubing, and I was amazed to see how water molecules moved across the membrane, demonstrating the power of osmosis. This simple experiment ignited my passion for exploring the intricacies of cellular processes, and I believe understanding these concepts is crucial for anyone interested in biology, chemistry, or the human body.
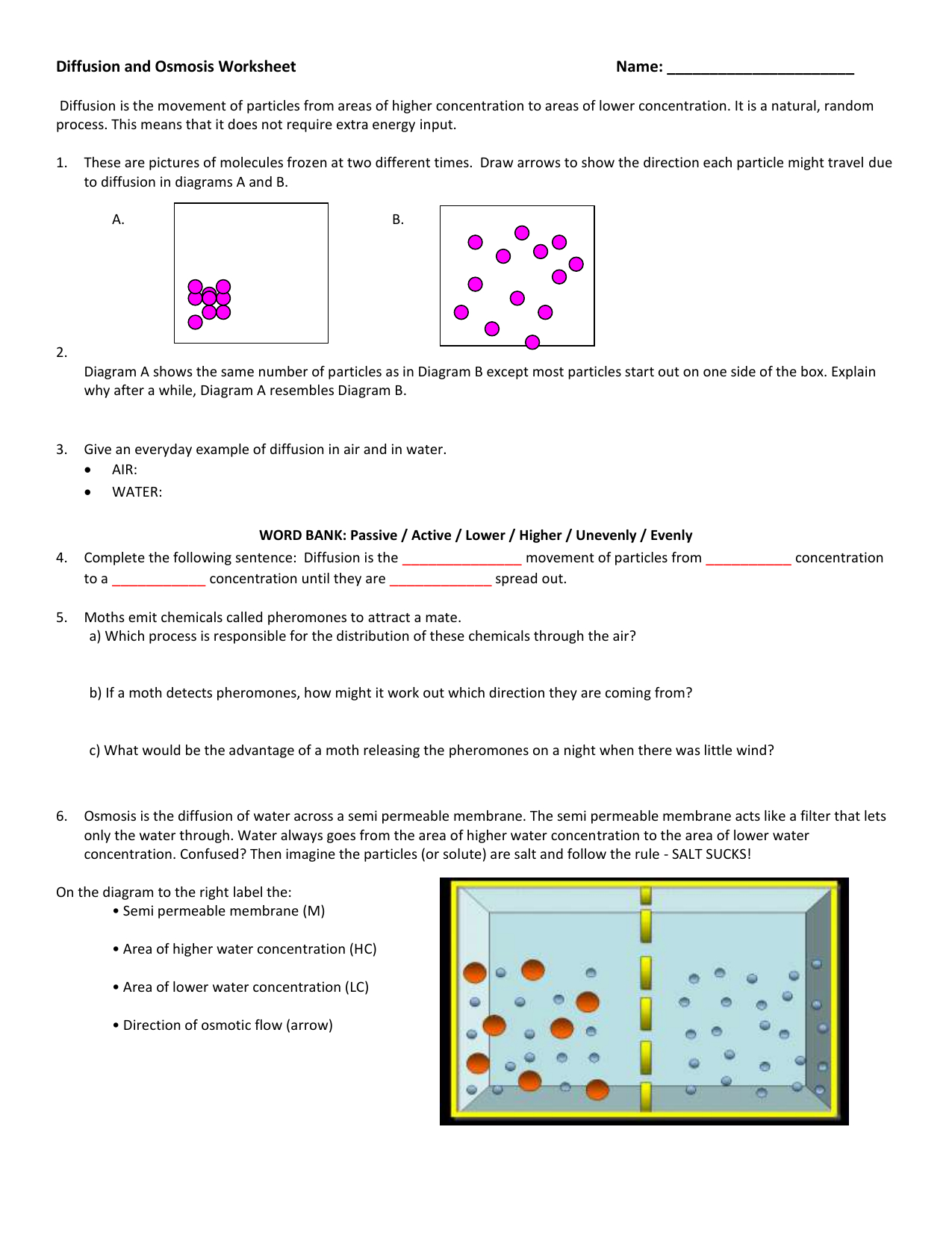
Image: db-excel.com
Today, we’ll delve deeper into the concepts of diffusion and osmosis, discussing their importance in various biological systems. We’ll explore how these processes contribute to the life-sustaining functions of organisms, from single-celled bacteria to complex multicellular organisms like ourselves. Through a detailed analysis of practical labs and the answers to common questions, this article aims to provide you with a comprehensive understanding of these crucial biological principles.
Understanding Diffusion and Osmosis: The Basics
Diffusion and osmosis are two closely related processes that involve the movement of molecules across membranes. Both processes are driven by the second law of thermodynamics, which states that the entropy of an isolated system always increases. In simpler terms, molecules naturally tend to move from areas of high concentration to areas of low concentration, seeking to distribute themselves evenly.
Diffusion: The Movement of Molecules
Diffusion is the movement of molecules from an area of high concentration to an area of low concentration. This movement occurs due to the random motion of molecules, resulting in a net movement down the concentration gradient. Imagine dropping a drop of food coloring into a glass of water. Over time, the food coloring will spread throughout the water, eventually reaching a state of uniform distribution. This is an example of diffusion in action.
Diffusion plays a vital role in various biological processes, including:
- Gas exchange in the lungs: Oxygen diffuses from the alveoli (tiny air sacs in the lungs) into the blood, while carbon dioxide diffuses from the blood into the alveoli to be exhaled.
- Nutrient transport: Nutrients like glucose diffuse from the small intestine into the bloodstream, delivering energy to the body’s cells.
- Waste removal: Waste products like carbon dioxide and urea diffuse from cells into the bloodstream, eventually being excreted from the body.
Osmosis: The Movement of Water
Osmosis is a special case of diffusion where the movement of water molecules across a selectively permeable membrane is driven by a difference in water potential. A selectively permeable membrane allows some molecules to pass through but blocks others. In osmosis, water molecules move from a region of high water potential (high concentration of water molecules) to a region of low water potential (low concentration of water molecules).
Imagine placing a red blood cell in a hypotonic solution (a solution with a lower solute concentration than the cell’s interior). Water molecules will move from the hypotonic solution into the red blood cell, causing it to swell and potentially burst. On the other hand, if you place a red blood cell in a hypertonic solution (a solution with a higher solute concentration than the cell’s interior), water molecules will move out of the cell into the hypertonic solution, causing the cell to shrink and shrivel. This difference in water potential is crucial for maintaining proper cell function and hydration in living organisms.

Image: athensmutualaid.net
Understanding the Role of Diffusion and Osmosis in Biological Systems
Diffusion and osmosis are essential for the proper functioning of all living organisms. They play a crucial role in:
- Cell transport: Diffusion and osmosis move essential nutrients, enzymes, and waste products across cell membranes, ensuring proper cell function.
- Maintaining cell volume: Osmosis helps regulate the amount of water inside cells, preventing them from shrinking or bursting due to changes in the surrounding environment.
- Plant growth and development: Osmosis is responsible for water movement from the roots to the leaves, providing essential moisture for photosynthesis and other life processes.
- Animal physiology: Diffusion and osmosis are involved in the regulation of blood pressure, the absorption of nutrients from the digestive system, and the elimination of waste products from the body.
Diffusion and Osmosis Lab: A Practical Exploration
A common experiment to demonstrate diffusion and osmosis involves using dialysis tubing, a selectively permeable membrane similar to the cell membrane. The experiment typically involves filling the dialysis tubing with a solution (e.g., starch solution) and submerging it in a beaker of water. The starch molecules are too large to pass through the dialysis tubing, while the water molecules are small enough to move across the membrane.
Here’s a breakdown of the lab procedure and expected observations:
Diffusion Lab Procedure
- Prepare the dialysis tubing: Soak the dialysis tubing in water for about 30 minutes to make it more permeable.
- Fill the tubing with solution: Fill the dialysis tubing with a solution (e.g., starch solution) and tie off the ends with string or rubber bands.
- Submerge the tubing: Submerge the filled dialysis tubing in a beaker of water.
- Observe and record changes: Observe the dialysis tubing over time and record any changes in its appearance or the surrounding water.
Expected Observations
- Diffusion of water: Water molecules will move across the dialysis tubing membrane into the tubing, causing it to swell.
- No diffusion of starch: The starch molecules will remain inside the dialysis tubing, as they are too large to pass through the membrane.
- Change in water potential: As water moves into the dialysis tubing, the water potential inside the tubing will increase, while the water potential outside the tubing will decrease.
Analyzing the Results
By analyzing the results of the experiment, students can understand the principles of diffusion and osmosis and how they relate to biological systems. The observations will demonstrate that water molecules can move across a selectively permeable membrane, leading to changes in concentration and water potential.
The lab provides a hands-on experience for students to visualize these concepts and develop a deeper understanding of how they influence biological processes.
Diffusion and Osmosis Lab PDF Answers: A Guide to Understanding the Concepts
Many students find it helpful to refer to lab PDFs, which can provide detailed instructions, background information, and answers to common lab questions. If you are working on a Diffusion and Osmosis lab, here are some of the most frequently asked questions and their answers that can help you better understand the concepts and analyze your results.
Frequently Asked Questions
1. What is the purpose of using a selectively permeable membrane in the diffusion and osmosis lab?
The selectively permeable membrane, in this case, dialysis tubing, mimics the behavior of the cell membrane. It allows the passage of certain molecules (like water) while restricting others (like starch) based on their size. This demonstrates how the cell membrane controls the movement of substances in and out of the cell, essential for various cellular processes.
2. Why does water move into the dialysis tubing in the osmosis experiment?
Water moves into the dialysis tubing because of the difference in water potential. The starch solution inside the tubing has a lower water potential than the pure water outside the tubing. Therefore, water molecules move from the region of high water potential (outside the tubing) to the region of lower water potential (inside the tubing), causing the tubing to swell.
3. Why doesn’t starch move out of the dialysis tubing in the diffusion experiment?
Starch molecules are too large to pass through the pores of the dialysis tubing membrane. The membrane acts as a barrier, preventing the passage of large molecules. This demonstrates how the cell membrane regulates the movement of molecules based on their size and shape, maintaining essential cellular balance.
4. What are the applications of diffusion and osmosis in the natural world?
Diffusion and osmosis are crucial for various biological processes, including nutrient uptake, waste removal, and maintaining cell volume, as discussed above. In the natural world, they are essential for processes like plant growth, the respiration of animals, and even the movement of substances within ecosystems, highlighting their significance in maintaining life on earth.
Tips and Expert Advice for Understanding Diffusion and Osmosis
By understanding the concepts of diffusion and osmosis, you can better comprehend the intricate processes that occur within living organisms. These concepts are essential for studying various biological topics, from cell biology to physiology, ecology, and even medicine. But mastering these concepts often requires a combination of theory and practical experience.
Here are some tips and advice for studying these processes:
- Visualize the concepts: Use diagrams, animations, and real-life examples to visualize the movement of molecules in diffusion and osmosis. This can help you grasp the fundamental principles better.
- Practice lab experiments: Conducting hands-on experiments like the dialysis tubing experiment provides a practical understanding of the concepts and reinforces your theoretical knowledge.
- Relate the concepts to everyday life: Think about how diffusion and osmosis work in everyday life, such as the scent of coffee spreading through a room or how plants absorb water from the soil.
- Use online resources: Many online resources, including video lectures, interactive simulations, and articles, can provide comprehensive explanations and examples of diffusion and osmosis.
- Review past lab reports: Examining lab reports from previous experiments focusing on diffusion and osmosis can help you understand the process and interpret your own results.
Diffusion And Osmosis Lab Pdf Answers
Conclusion
Understanding diffusion and osmosis is crucial for anyone interested in biology, chemistry, or the human body. These processes are essential for maintaining life and play a crucial role in various biological functions, from cell transport to plant growth and animal physiology. By conducting labs and exploring these concepts further, you can gain a deeper understanding of the fundamental principles that govern life itself.
Are you interested in learning more about diffusion and osmosis? What other biological processes pique your curiosity? Let’s continue this fascinating exploration together!






