Have you ever wondered how much air you need to completely burn a specific amount of fuel? Or maybe you’ve been curious about the volume of gas produced during a chemical reaction? These questions, and many others, are at the heart of gas stoichiometry, a fundamental concept in chemistry that allows us to predict and understand the relationships between reactants and products in chemical reactions involving gases. It’s not just an abstract academic concept, it plays a crucial role in various fields, from designing engines to understanding atmospheric processes.

Image: printablelibfriedmann.z13.web.core.windows.net
So, how can we navigate the world of gas stoichiometry and its intricate calculations? The answer lies in the power of worksheets! In this article, we’ll delve deeper into the world of gas stoichiometry, exploring its key principles and equipping you with a valuable resource – a comprehensive gas stoichiometry worksheet with answers – to solidify your understanding and master this essential topic.
The Foundation of Gas Stoichiometry: A Journey into the World of Gases
Imagine a balloon slowly filling with air. As you add more air, the balloon expands, and the pressure inside increases. This illustrates a key characteristic of gases – their volume and pressure are directly related. Unlike solids and liquids, gases fill their container completely, and their volume is highly sensitive to changes in temperature and pressure.
Gas stoichiometry builds upon these fundamental properties, utilizing the Ideal Gas Law, a powerful equation that describes the relationship between the pressure (P), volume (V), temperature (T), and number of moles (n) of a gas:
PV = nRT
Where R is the ideal gas constant. This equation is the cornerstone of gas stoichiometry calculations, allowing us to relate the volume of gases involved in a chemical reaction to the moles of reactants and products.
Mastering the Art of Gas Stoichiometry: A Comprehensive Worksheet
Navigating the world of gas stoichiometry can feel overwhelming at first, but with the right tools and guidance, it can become a breeze. To make your journey smoother, we’ve created a comprehensive gas stoichiometry worksheet with detailed answers. This worksheet provides a structured approach to solving various problems, covering a wide range of scenarios. It’s designed to reinforce your understanding of key concepts and equip you with the skills to tackle any gas stoichiometry problem.
Here are some key areas covered in the worksheet:
-
Converting between moles and volumes of gases: This section walks you through the process of converting between moles and volumes of gases using the Ideal Gas Law. Imagine you know the volume of a gas at a specific temperature and pressure, but need to find the number of moles. The worksheet will guide you step-by-step through the calculations.
-
Calculating the volume of a gas produced or consumed in a reaction: Chemical reactions involve the transformation of reactants into products, often accompanied by the release or consumption of gases. The worksheet provides examples and solutions for calculating the volume of gases produced or consumed in specific reactions.
-
Stoichiometry calculations involving gases at STP: Standard temperature and pressure (STP) provide a convenient reference point for gas stoichiometry calculations. You’ll learn how to convert volumes of gases at non-STP conditions to STP, simplifying calculations.
-
Determining the limiting reactant in a reaction involving gases: Sometimes, one reactant is completely consumed before the other, limiting the amount of product formed. The worksheet will help you identify the limiting reactant in reactions involving gases, enabling you to accurately predict the theoretical yield of the reaction.
Beyond the Worksheet: Real-World Applications
Gas stoichiometry isn’t just a theoretical concept; it has profound implications for our everyday lives. This powerful tool finds its applications in diverse fields, such as:
-
Automotive Industry: The combustion of fuels in engines relies on gas stoichiometry principles. Engineers use these principles to optimize fuel efficiency and minimize emissions.
-
Environmental Science: Understanding gas stoichiometry is crucial for mitigating atmospheric pollution. For example, scientists use it to analyze the production and consumption of greenhouse gases like CO2.
-
Chemical Engineering: Gas stoichiometry governs many industrial processes, enabling chemical engineers to design and optimize chemical processes involving gases.
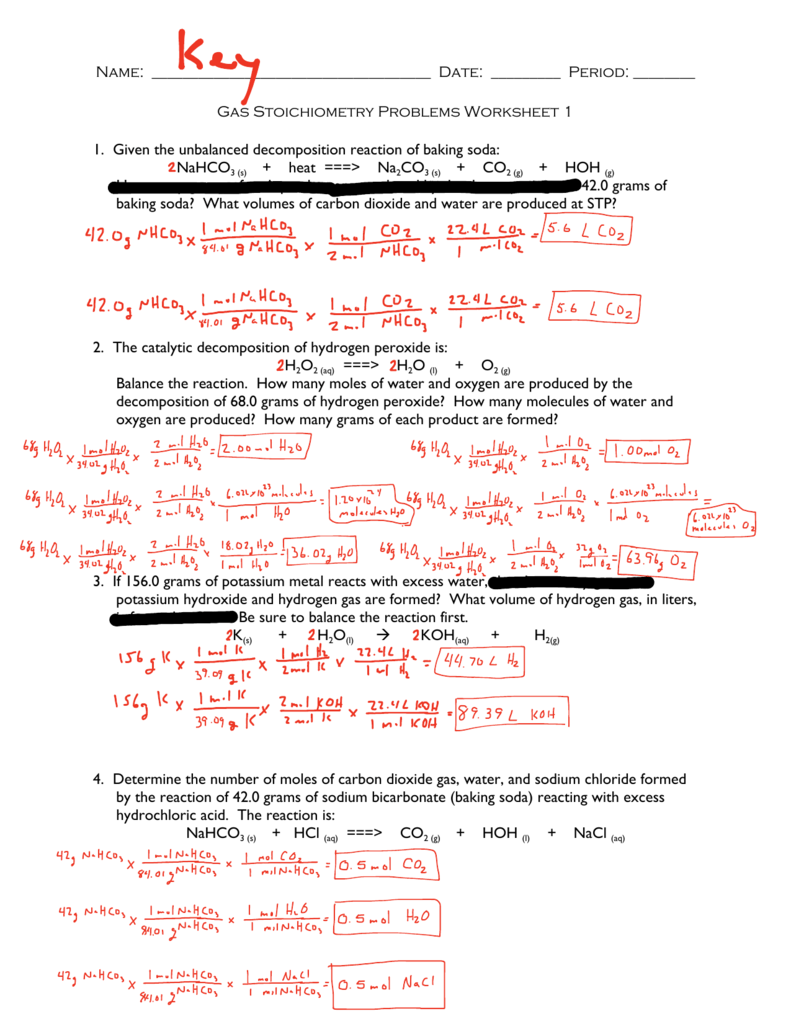
Image: gmbar.co
Gas Stoichiometry Worksheet With Answers Pdf
Tips for Success: Mastering Gas Stoichiometry
Mastering gas stoichiometry requires a blend of conceptual understanding and problem-solving skills. Here are some tips to help you excel in this area:
-
Practice, Practice, Practice: As with any skill, consistency is key. Solve as many problems as you can, starting from simpler ones and gradually progressing to more complex scenarios.
-
Visualize the Concepts: Draw diagrams and visualize the reaction processes. This can help you gain a deeper understanding and make the calculations more intuitive.
-
Seek Assistance: Don’t hesitate to seek assistance from your teacher, classmates, or online resources if you encounter difficulties.
Conclusion:
Gas stoichiometry is a fundamental concept in chemistry that empowers us to understand and predict the behavior of gases in chemical reactions. The gas stoichiometry worksheet with answers is an invaluable resource that can help you solidify your understanding of this essential topic, providing a structured approach to solving a wide range of problems. Remember, practice, visualization, and seeking help are key to unlocking the secrets of gas stoichiometry.
Now, let’s embark on this exciting journey, equip yourself with the gas stoichiometry worksheet, and discover the fascinating world of gases!






