Have you ever wondered what holds atoms together to form the countless substances that make up our world? From the water we drink to the air we breathe, the very fabric of reality is built upon the unseen forces of chemical bonding. Two of the most fundamental types of chemical bonds are ionic and covalent bonds, which govern how atoms interact and share electrons. Understanding these bonds is crucial for comprehending the properties and behaviors of all matter around us.
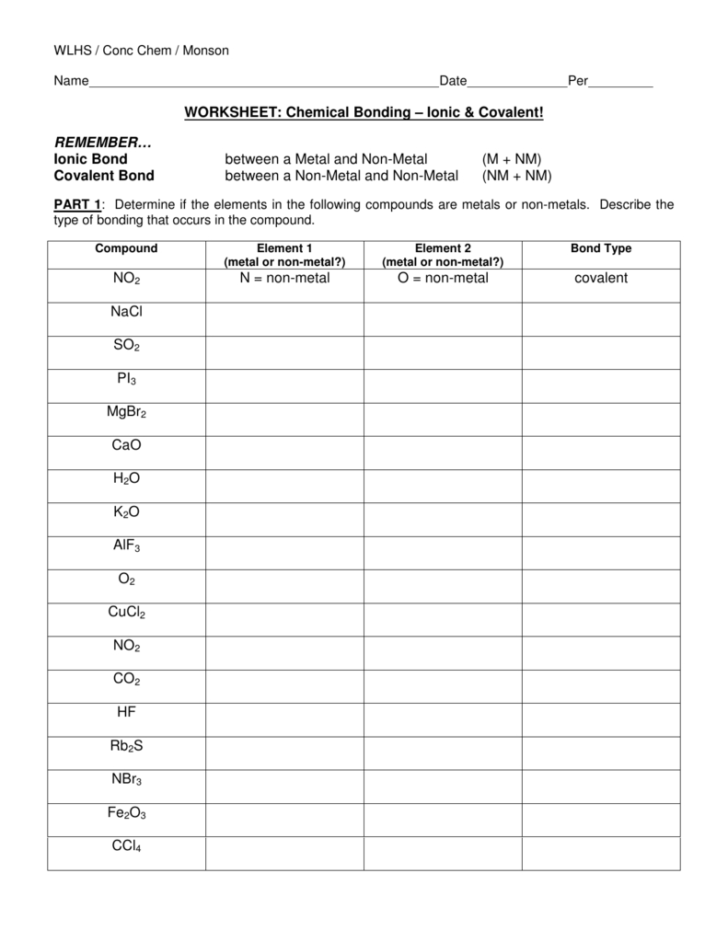
Image: studyzoneqatbuttressed.z13.web.core.windows.net
This comprehensive guide delves into the fascinating world of ionic and covalent bonds, exploring their formation, characteristics, and real-world applications. We’ll embark on a journey through the microscopic realm of atoms and molecules, revealing the secrets behind the fundamental building blocks of our universe. Prepare to unravel the mysteries of chemical bonding and gain a deeper appreciation for the complexities and elegance of the natural world.
Delving into the Core of Chemical Bonding
At the heart of chemical bonding lies the concept of electronegativity, a measure of an atom’s ability to attract electrons towards itself. This fundamental force dictates how atoms interact and form bonds. When two atoms with significantly different electronegativities come together, one atom (the more electronegative one) tends to pull electrons away from the other, forming an ionic bond. Conversely, when atoms with similar electronegativities interact, they share electrons to achieve a stable configuration, resulting in a covalent bond.
Ionic Bonding: A Dance of Opposite Charges
Imagine two atoms with contrasting tendencies: one eager to lose electrons and the other eager to gain them. This scenario is the foundation of ionic bonding. A prime example is the interaction between sodium (Na) and chlorine (Cl) to form sodium chloride (NaCl), commonly known as table salt. Sodium readily loses its outer electron, becoming a positively charged ion (Na+), while chlorine readily gains an electron, becoming a negatively charged ion (Cl-).
These oppositely charged ions are attracted to each other, forming an electrostatic force known as an ionic bond. The resulting compound, sodium chloride, is a crystalline solid held together by the strong attraction between the positively charged sodium ions and the negatively charged chloride ions. This attraction is so strong that ionic compounds have high melting and boiling points, demonstrating the stability of the ionic bond.
Key Characteristics of Ionic Bonds:
- Occur between metals and nonmetals.
- Involve the transfer of electrons.
- Result in the formation of ions (charged atoms).
- Exhibit strong electrostatic attraction between oppositely charged ions.
- Typically form crystalline solids with high melting and boiling points.
- Readily dissolve in polar solvents like water, allowing for ion mobility.
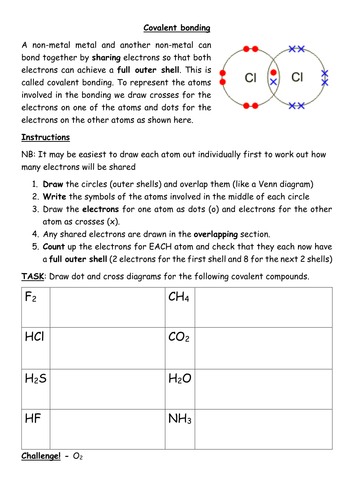
Image: lessoncampusbaecker.z13.web.core.windows.net
Covalent Bonding: Sharing the Love of Electrons
In contrast to ionic bonds, covalent bonds arise from the sharing of electrons between atoms. This occurs when atoms have similar electronegativities, leading to a more balanced distribution of electrons. Consider the molecule of water (H2O). Hydrogen (H) and oxygen (O) readily share electrons to form two covalent bonds, each involving a pair of shared electrons.
Atoms involved in covalent bonding achieve a stable electron configuration by sharing electrons and achieving a full outer shell. The shared electrons are attracted to the nuclei of both atoms, creating a force that holds the atoms together in a covalent bond. This bond is generally stronger than intermolecular forces (forces between molecules), as it involves a direct sharing of electrons.
Key Characteristics of Covalent Bonds:
- Occur between nonmetals.
- Involve the sharing of electrons.
- Result in the formation of molecules.
- Exhibit a range of bond strengths depending on the nature of the atoms involved.
- Can form single, double, or triple bonds depending on the number of shared electrons.
- Typically exist in gases, liquids, or solids with varying melting and boiling points.
Exploring Real-World Applications
Chemical bonding is not just a theoretical concept; it plays a crucial role in countless aspects of our daily lives. From the medicines we take to the materials we use, the knowledge of ionic and covalent bonds is fundamental to understanding the world around us.
Ionic Compounds:
- Table Salt (NaCl): A common ionic compound essential for human health and used in food preservation.
- Calcium Carbonate (CaCO3): A key component of seashells, limestone, and chalk, used in construction and agricultural applications.
- Sodium Bicarbonate (NaHCO3): Commonly known as baking soda, used in baking, cleaning, and as an antacid.
- Potassium Chloride (KCl): A vital electrolyte for maintaining fluid balance and nerve function in the body.
Covalent Compounds:
- Water (H2O): The essential solvent of life, participating in biological processes and facilitating chemical reactions.
- Carbon Dioxide (CO2): A greenhouse gas crucial for plant photosynthesis and a major component of the Earth’s atmosphere.
- Glucose (C6H12O6): A vital energy source for living organisms, used in cellular respiration to produce energy.
- Ethanol (C2H5OH): A common alcohol found in beverages and used as a solvent and fuel.
Bridging the Gap Between Theory and Practice: Worksheets as Tools for Learning
Chemical bonding worksheets are invaluable tools for understanding and applying the concepts of ionic and covalent bonds. These worksheets provide students with hands-on experience, allowing them to practice solving problems, predicting bond types, and drawing Lewis dot structures. By working through these problems, students solidify their understanding of the fundamental principles of chemical bonding.
Benefits of Using Worksheets:
- Reinforce Learning: Worksheets provide repeated practice, helping students remember key concepts and apply them in different contexts.
- Develop Critical Thinking Skills: Solving problems on worksheets encourages students to think critically, analyze information, and apply their knowledge to solve specific scenarios.
- Enhance Conceptual Understanding: Visual representations and problem-solving exercises in worksheets promote a deeper understanding of the underlying principles of chemical bonding.
- Identify Learning Gaps: Worksheets allow teachers to identify areas where students may need further support or clarification.
Navigating the World of Chemical Bonding Worksheets: A Comprehensive Guide
Many resources are available online and in textbooks to help students practice and enhance their understanding of chemical bonding. Here’s a guide to navigating the world of chemical bonding worksheets:
Understanding the Basics:
- Start with the fundamentals of electronegativity, ions, and molecules.
- Practice drawing Lewis dot structures to represent the sharing or transfer of electrons.
- Familiarize yourself with the key characteristics of ionic and covalent bonds.
Progressing to Complex Concepts:
- Work through worksheets that involve predicting bond types based on the electronegativity differences between atoms.
- Explore worksheets that focus on the properties of ionic and covalent compounds, such as melting points, boiling points, and solubility.
- Challenge yourself with worksheets that involve writing chemical formulas for ionic and covalent compounds.
Don’t Forget the Real World:
- Find worksheets that link chemical bonding concepts to real-life applications, such as the properties of common materials or the mechanisms of biochemical reactions.
- Explore worksheets that involve analyzing experimental data or interpreting graphs related to chemical bonding.
Chemical Bonding Ionic And Covalent Worksheet
Conclusion
Unveiling the intricacies of chemical bonding, particularly ionic and covalent bonds, is an exciting journey that opens doors to a deeper understanding of the universe around us. These bonds not only shape the structure and properties of matter but also underpin the very foundations of life itself. Through the use of effective learning tools like chemical bonding worksheets, students can strengthen their understanding, develop critical thinking skills, and gain valuable knowledge applicable across various fields. So, dive into the world of chemical bonding, explore the wonders of ionic and covalent interactions, and reap the rewards of a more informed and curious mind.






