Have you ever wondered why a balloon inflates when you blow into it, but deflates when you take it to a higher altitude? Or why a scuba diver needs to be careful about how much air they breathe at different depths? These seemingly simple phenomena are all governed by a fundamental scientific principle: Boyle’s Law. This law, discovered by the brilliant Robert Boyle in the 17th century, describes the inverse relationship between the pressure and volume of a gas.

Image: gambr.co
In this article, we’ll embark on a fascinating journey into the world of Boyle’s Law, using the interactive PHET simulation as our guide. This simulation provides a unique and engaging way to visualize and experience the principles of Boyle’s Law firsthand, making it an invaluable tool for students, educators, and anyone curious about the wonders of science.
Navigating the PHET Simulation: A Hands-On Understanding of Boyle’s Law
The PHET simulation on Boyle’s Law is a virtual laboratory, allowing us to manipulate variables and observe the effects in real-time. Imagine a box with a piston, representing a closed container filled with gas. You can adjust the piston’s position, changing the volume of the container. The simulation then calculates the pressure of the gas, revealing the intricate relationship between these two variables.
Let’s dive into the key aspects of the simulation:
Experiment 1: The Inverse Relationship
Start by setting the piston to a specific position, representing a set volume. Observe the pressure reading. Now, slowly move the piston inwards, decreasing the volume. You’ll witness the pressure gauge rise significantly. This demonstrates the inverse relationship between pressure and volume. The smaller the volume, the higher the pressure, and vice versa.
Experiment 2: The Impact of Temperature
The simulation also allows you to explore the effect of temperature on gas pressure. Increase the temperature of the gas while keeping the volume constant. Observe how the pressure gauge rises. This illustrates the direct relationship between temperature and pressure. As temperature increases, the gas molecules move faster, colliding with the container walls more frequently, leading to higher pressure.
Experiment 3: Real-World Applications
The PHET simulation extends beyond theoretical concepts. You can explore real-world applications of Boyle’s Law. Imagine a bicycle pump, where you compress air to inflate the tire. Or think about a scuba diver descending into deeper water, where pressure increases with depth. Boyle’s Law comes into play in these situations, explaining the mechanics of how pressure changes affect the volume of gases.
Dissecting the Mathematics: The Equation Behind Boyle’s Law
Boyle’s Law can be expressed mathematically, providing a concise and powerful tool for understanding the relationship between pressure and volume. The equation states that the product of pressure (P) and volume (V) is constant for a given mass of gas at a constant temperature.
P1 V1 = P2 V2
This equation signifies that if the pressure of a gas doubles, the volume halves, and vice versa, provided the temperature remains constant.
Beyond the Simulation: Real-World Encounters with Boyle’s Law
Beyond the virtual laboratory of the PHET simulation, Boyle’s Law governs numerous real-world phenomena. Here are some examples:
- Breathing: When you inhale, you expand your chest cavity, increasing the volume of your lungs. This decrease in pressure draws air into your lungs, following Boyle’s Law.
- Aerosol Cans: The pressure inside an aerosol can is a result of Boyle’s Law. When you spray the can, you release the pressure, causing the contents to expand and exit.
- Automotive Engines: The internal combustion engine relies on Boyle’s Law to draw air into the cylinders during the intake stroke. The compression of air in the cylinder subsequently increases pressure, which contributes to the combustion process.
- Medicine: In medical settings, Boyle’s Law finds applications in various therapies, such as ventilation and oxygen delivery. By controlling pressure and volume, medical professionals can regulate the delivery of gases.
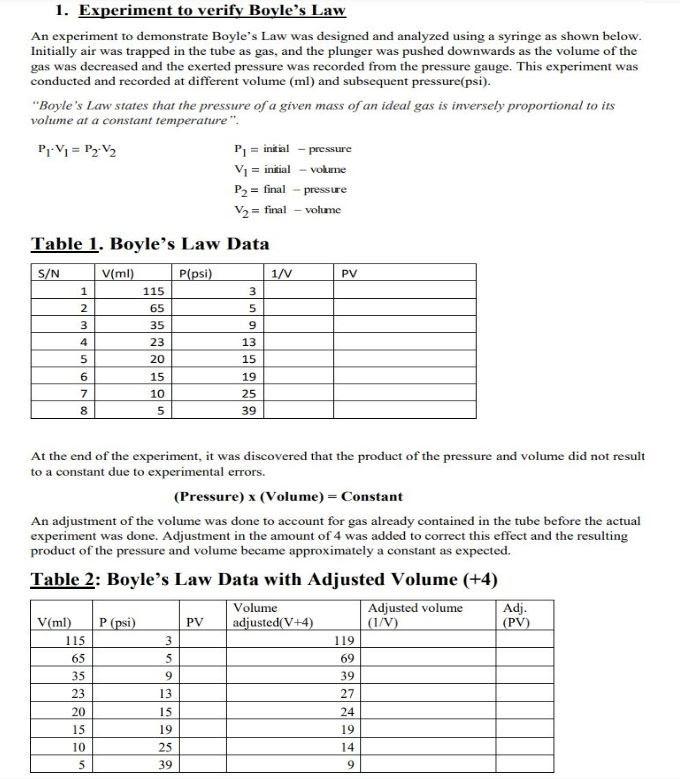
Image: www.chegg.com
Unlocking the Potential: Leveraging Boyle’s Law in Your Life
Understanding Boyle’s Law goes beyond mere academic knowledge. It empowers us to understand and interact with our environment in a new light. For instance, when hiking in mountainous terrain, we can better appreciate the decrease in air pressure at higher altitudes and its implications for respiration. Moreover, an understanding of Boyle’s Law can help us make informed decisions when dealing with pressurized containers or systems.
Boyle’S Law Phet Simulation Answer Key Pdf
Conclusion: A Journey of Scientific Discovery
The journey through the PHET simulation, coupled with a deeper exploration of Boyle’s Law, reveals the beauty and practicality of scientific principles. Through observation, experimentation, and a little bit of mathematical insight, we gain a profound understanding of how pressure and volume interact in a wide array of contexts. From everyday phenomena to complex engineering applications, Boyle’s Law underscores the fundamental laws that govern our physical world.
We invite you to explore further, continue experimenting with the PHET simulation, and delve into the fascinating realm of physics and chemistry. The journey of scientific discovery is a continuous one, and understanding Boyle’s Law is a crucial step in that journey.






